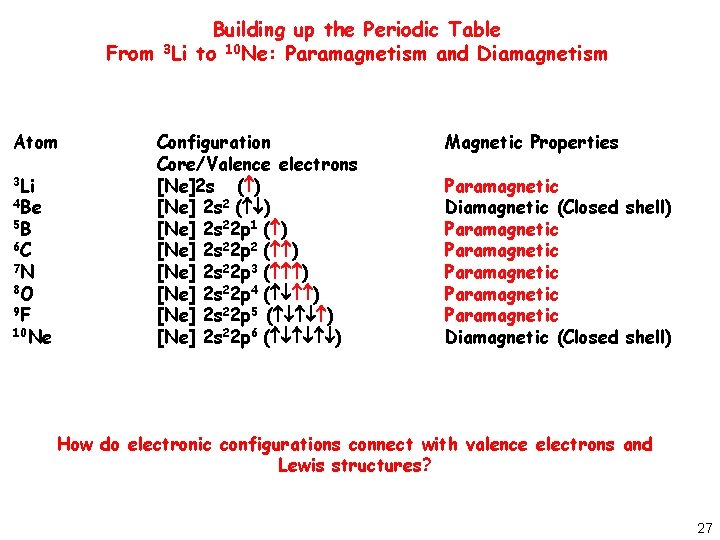
A very simple concept elements having even number of total electrons except 10 like Neon and 16 like O2 are diamagnetic and elements having odd no. They can be solid liquid or gas.
 Transition Metals Introduction To Chemistry
Transition Metals Introduction To Chemistry
Atoms with all diamagnetic electrons are called diamagnetic atoms.
Paramagnetic vs diamagnetic periodic table. How to Tell if a Substance is Paramagnetic or Diamagnetic. Compounds are diamagnetic when they contain no unpaired electrons. For example from their position on the periodic table we easily recognize them as metals semimetals metalloids or nonmetals.
They can be solid liquid or gas. Above curie point a ferromagnetic substance becomes ferromagnetic. If it shows unpaired electrons then the substance is paramagnetic.
The magnitude of the paramagnetism is expressed as an effective magnetic moment μ eff. Typical magnetic susceptibilities for diamagnetic materials are in the region of 10 5. Molecular compounds that contain one or more unpaired electrons are paramagnetic.
The magnetic form of a substance can be determined by examining its electron configuration. The rest are metalloids o nonmetals - and inert gases i on the top right hand of the long periodic table. 15082020 The periodic table is a convenient way to correlate chemical properties.
Most of the elements in the periodic table are diamagnetic. This is mainly because the paramagnetic materials have unpaired electrons whereas diamagnetic materials have none of their electrons unpaired. With the rise of temperature a parmagnetic substance becomes diamagnetic.
30112013 The term paramagnetic refers to the attraction of a material to an external magnetic field while the term diamagnetic refers to the repulsion of a material from an external magnetic field. Most elements are metals M. The magnetic moment of every atom of diamagnetic substancematerial is.
The electron pairs in the diamagnetic materials are linearly aligned under the application of the applied magnetic field. If a substance does not possess any paramagnetic or ferromagnetic it means that it is diamagnetic. 22 Paramagnetism Although we stated above that all materials exhibit some diamagnetism this may be negligible compared to a positive magnetic susceptibility arising from the magnetic moments of unpaired electrons aligning themselves with the applied eld.
A paramagnetic electron is an unpaired electron. Low 0 r. 0 SpinMagnetic momentDipole alignment.
This process can be broken into four steps. Of electrons and also with 10 and 16 no. 0 Low positive χ.
Every atom is a magnetic dipole having a resultant magnetic moment. Everything else is paramagnetic. It means that it repels or opposes the magnetic field.
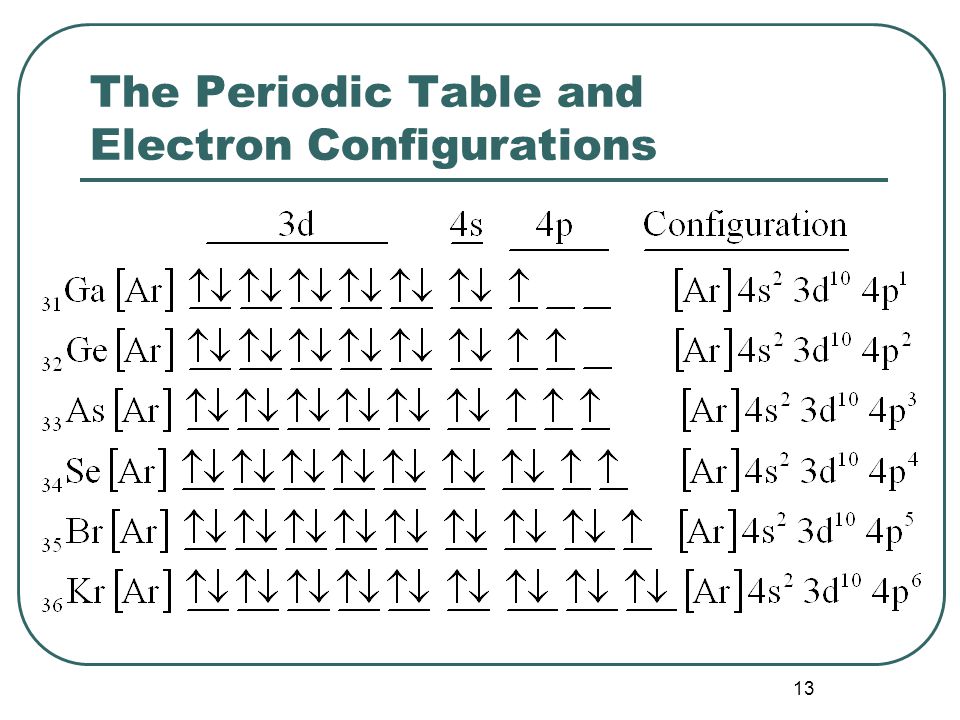
Of electrons are paramagnetic in nature. It shows you how to identify if an element is paramagnetic or diamagnetic by writin. All electrons are paired in the atom Can determine from periodic table - if element is located at the end of a subshell then it is diamagnetic.
Find the electron configuration. This chemistry video tutorial focuses on paramagnetism and diamagnetism. An atom could have ten diamagnetic electrons but as long as it also has one paramagnetic electron it is still considered a paramagnetic atom.
If all electrons are paired the substance is diamagnetic. Visit my youtube channel chemist addiction. 0 High positive χ.
An atom is considered paramagnetic if even one orbital has a net spin. Not all electrons are paired in the atom Diamagnetic.
 Paramagnetism And Diamagnetism Video Khan Academy
Paramagnetism And Diamagnetism Video Khan Academy
 Describe Magnetic Properties Of D Block Elements Qs Study
Describe Magnetic Properties Of D Block Elements Qs Study
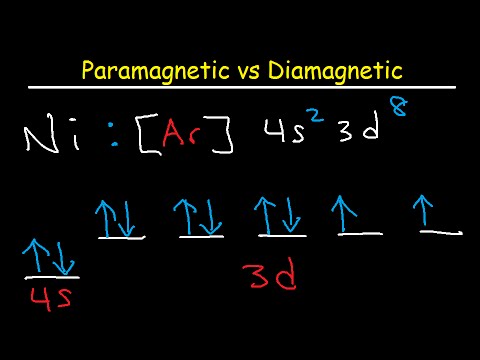 Paramagnetic Vs Diamagnetic Paired Vs Unpaired Electrons Electron Configuration Youtube
Paramagnetic Vs Diamagnetic Paired Vs Unpaired Electrons Electron Configuration Youtube
Http Www Physics Usu Edu Peak Phys 2710 Mn Mn 9 Pdf
 1 Paramagnetism And Diamagnetism Atoms With Unpaired Electrons Are Called Paramagnetic Paramagnetic Atoms Are Attracted To A Magnet Diamagnetic Atoms Ppt Download
1 Paramagnetism And Diamagnetism Atoms With Unpaired Electrons Are Called Paramagnetic Paramagnetic Atoms Are Attracted To A Magnet Diamagnetic Atoms Ppt Download
 What Is The Meaning Of The Term Paramagnetic Greenwood Magnetics
What Is The Meaning Of The Term Paramagnetic Greenwood Magnetics
 Paramagnetic Periodic Table Page 1 Line 17qq Com
Paramagnetic Periodic Table Page 1 Line 17qq Com
Http Personal Strath Ac Uk Barry Williams Book Chapter 2032x Pdf
 Chemistry Periodic Variations 22 Of 23 Diamagnetic Atoms And Ions Youtube
Chemistry Periodic Variations 22 Of 23 Diamagnetic Atoms And Ions Youtube
 Chapter 17 Manyelectron Atoms And Chemical Bonding 17
Chapter 17 Manyelectron Atoms And Chemical Bonding 17
 Advances In Magnetic Materials
Advances In Magnetic Materials
 Chemistry Periodic Variations 23 Of 23 Paramagnetic Atoms And Ions Youtube
Chemistry Periodic Variations 23 Of 23 Paramagnetic Atoms And Ions Youtube
What Are Examples Of Diamagnetic Elements Quora
 Paramagnetic Periodic Table Page 1 Line 17qq Com
Paramagnetic Periodic Table Page 1 Line 17qq Com
 Identifying Elements That Are Paramagnetic Or Diamagnetic Youtube
Identifying Elements That Are Paramagnetic Or Diamagnetic Youtube
 Consider The Elements Na Mg Al Si P W Clutch Prep
Consider The Elements Na Mg Al Si P W Clutch Prep
 Q What Causes Iron Nickel And Cobalt To Be Attracted To Magnets But Not Other Metals Ask A Mathematician Ask A Physicist
Q What Causes Iron Nickel And Cobalt To Be Attracted To Magnets But Not Other Metals Ask A Mathematician Ask A Physicist
 Understanding Paramagnetism Diamagnetism Youtube
Understanding Paramagnetism Diamagnetism Youtube
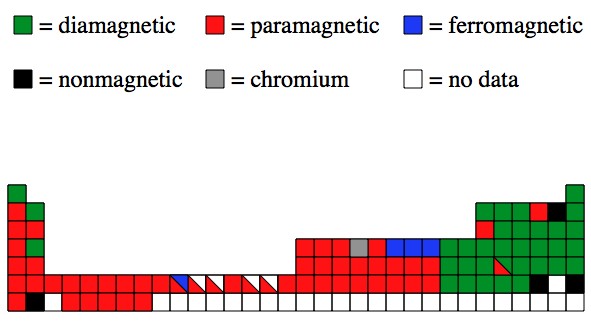
 Figure C 1 Periodic Table Showing The Type Of Magnetic Behavior Of Download Scientific Diagram
Figure C 1 Periodic Table Showing The Type Of Magnetic Behavior Of Download Scientific Diagram
