It also explains the bonding in a number of other molecules such as violations of the octet rule and more molecules with more complicated bonding beyond the scope of this text that are difficult to describe with Lewis structures. We can use the molecular orbital diagram to predict whether the molecule is paramagnetic or diamagnetic.
 Identifying Elements That Are Paramagnetic Or Diamagnetic Youtube
Identifying Elements That Are Paramagnetic Or Diamagnetic Youtube
One of the easiest methods to quickly solve Molecular Orbital TheoryMOT questions and understand the concept.
Paramagnetic vs. diamagnetic molecular orbital theory. It shows you how to identify if an element is paramagnetic or diamagnetic by writin. If the species has an even number of electrons it is likely diamagetic. 08082011 This video is a lesson on how MO theory is used to predict the magnetic properties of certain substances.
We now turn to a molecular orbital description of the bonding in ceO2. 07042019 Use molecular orbital theory to determine whether F22 is paramagnetic or diamagnetic and calculate its bond order. If it has unpaired electrons then the substance is paramagnetic.
A paramagnetic electron is an unpaired electron. 20092020 Molecular Oxygen is Paramagnetic. The correct explanation comes from Molecular Orbital theory.
04122017 About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy. It so happens that the molecular orbital description of this molecule provided an explanation for a long-standing puzzle that. Materials tend to show weak magnetic properties in the presence of an external magnetic field.
Is Ne2 2 paramagnetic or diamagnetic. Yet oxygen is paramagnetic. It shows that all the electrons in oxygen are paired so oxygen should be diamagnetic.
Explained with a few questionsCheck out prev. A substance is paramagnetic attracted to a magnet. This video is about the Molecular Orbital Theory and discusses in details the formation of diatomic molecules of the elements from periods 1 and 2 of the pe.
You can learn about it and its application to 2nd row elements here. Some materials get attracted to the external magnetic. Can someone help me.
The Central Science Chapter 9 Sectio. 11112014 Because there is only one electron in the π 2p x orbital and in the π 2p y orbital there are unpaired electrons and OF is paramagnetic. To understand this answer you have to know about molecular orbital MO theory of bonding.
30112013 The key difference between paramagnetic and diamagnetic materials is that the paramagnetic materials get attracted to external magnetic fields whereas the diamagnetic materials repel from the magnetic fields. If all the electrons are paired the molecule is diamagnetic. This page contains materials for the session on hybridization molecular orbitals and paramagnetism.
25092016 The Lewis structure of O2 gives a misleading impression. Paramagnetism is a result of unpaired electrons. If one or more electrons are unpaired the molecule is paramagnetic.
If all electrons in the particle are paired then the substance made of this particle is diamagnetic. Safety How YouTube works Test new features Press Copyright Contact us Creators. A simple rule of thumb is used in chemistry to determine whether a particle atom ion or molecule is paramagnetic or diamagnetic.
The atomic orbitals of the O atoms overlap to form the σ and π orbitals of the O2 molecule as shown in the diagram above. I have F22 as paramagnetic but I dont know how to calculate the bond order. If the species has an odd number of electrons it must be paramagnetic.
An atom is considered paramagnetic if even one orbital has a net spin. NO 56-1 10 valence electrons σ2s 2 σ 2s 2 π2p x 2 π2p y 2 σ2p z 2 Here all of the electrons are paired so NO is diamagnetic and the answer is B. It features a 1-hour lecture video and also presents the prerequisites learning objectives reading assignment lecture slides homework with solutions and resources for.
Whenever two electrons are paired together in an orbital or their total spin is 0 they are diamagnetic electrons. Molecular orbital theory MO theory provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Atoms with all diamagnetic electrons are called diamagnetic atoms.
This chemistry video tutorial focuses on paramagnetism and diamagnetism. If we count the valence electrons available to the four species you name we can take a pretty quick guess as to what might be paramagnetic.
Is Sulphur Dioxide Paramagnetic Or Diamagnetic Quora
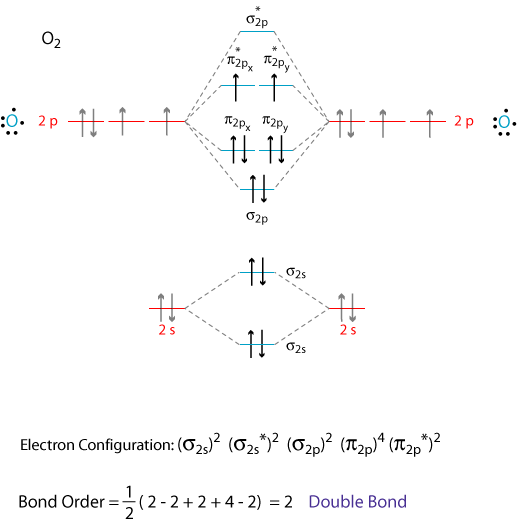 O2 Is Paramagnetic Or Diamagnetic Socratic
O2 Is Paramagnetic Or Diamagnetic Socratic
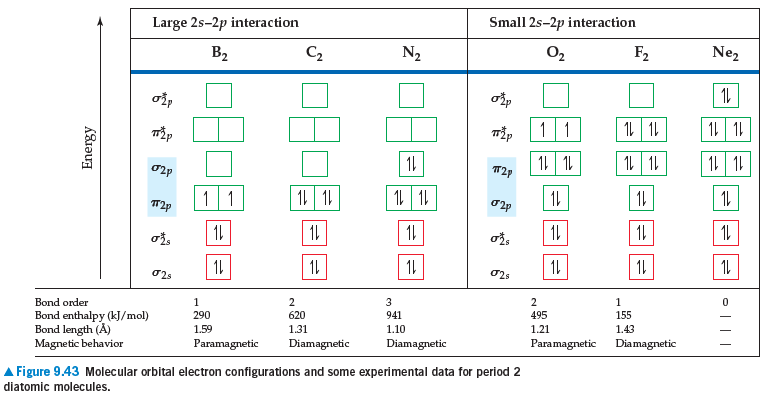 Answered N Atomic Orbitals No Molecular Orbitals Bartleby
Answered N Atomic Orbitals No Molecular Orbitals Bartleby
 Using The Mo Diagram Of No Calculate The Bond Order Compare It To No Socratic
Using The Mo Diagram Of No Calculate The Bond Order Compare It To No Socratic
Is O2 Paramagnetic Or Diamagnetic Quora
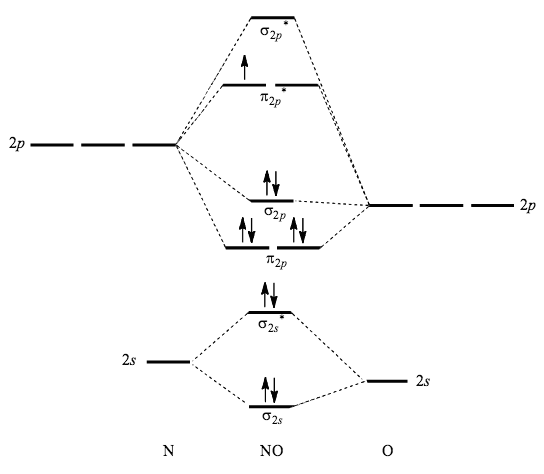 Using The Mo Diagram Of No Calculate The Bond Order Compare It To No Socratic
Using The Mo Diagram Of No Calculate The Bond Order Compare It To No Socratic
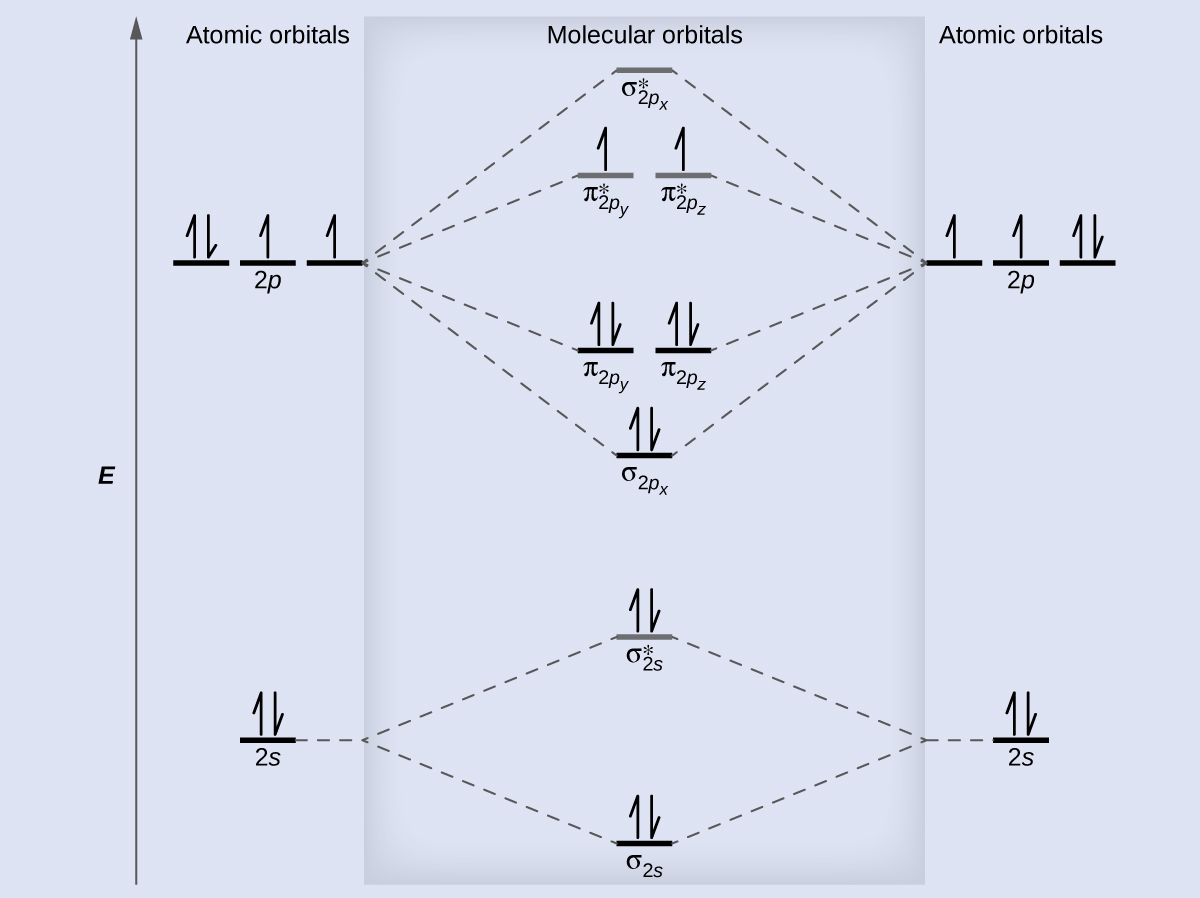 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic Study Com
Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic Study Com
Is Ne2 A Paramagnetic Or Diamagnetic Quora
 Based On The Mo Diagrams For O 2 O 2 And O 2 Answer The Following 1 Is O 2 Paramagnetic Or Diamagnetic 2 Which Will Have The Shortest Bond Length 3 Which Will Have The
Based On The Mo Diagrams For O 2 O 2 And O 2 Answer The Following 1 Is O 2 Paramagnetic Or Diamagnetic 2 Which Will Have The Shortest Bond Length 3 Which Will Have The
 Paramagnetic Vs Diamagnetic Paired Vs Unpaired Electrons Electron Configuration Youtube
Paramagnetic Vs Diamagnetic Paired Vs Unpaired Electrons Electron Configuration Youtube
 Is Be2 Diamagnetic Chemistry Stack Exchange
Is Be2 Diamagnetic Chemistry Stack Exchange
 Molecular Orbital Theory Bond Order Diamagnetic Vs Paramagnetic Youtube
Molecular Orbital Theory Bond Order Diamagnetic Vs Paramagnetic Youtube
 Why Is Diboron B2 Paramagnetic Chemistry Stack Exchange
Why Is Diboron B2 Paramagnetic Chemistry Stack Exchange
 Why Is Oxygen Paramagnetic Chemistry Stack Exchange
Why Is Oxygen Paramagnetic Chemistry Stack Exchange
 Molecular Orbital Theory Bond Order Diamagnetic Vs Paramagnetic Youtube
Molecular Orbital Theory Bond Order Diamagnetic Vs Paramagnetic Youtube

